Patanjali Ayurved CEO on Tuesday said the firm gave all the information about its newly launched Covid-19 medicine to Ministry of AYUSH. On Twitter, Acharya Balkrishna said, “communication gap has been done away with”.
“This govt provides encouragement and pride to Ayurveda. The communication gap has been done away with and we have 100% fulfilled all standard parameters for Randomised Placebo-Controlled Clinical Trials. We’ve given info for the same to Ministry of AYUSH,” Balkrishna said on Twitter.
The AYUSH ministry on Tuesday asked yoga guru Ramdev’s Patanjali Ayurved to provide “at the earliest” the composition and other details of the medicine it claimed is for the treatment of COVID-19, and ordered the firm to stop advertising the product until the “issue” is examined.
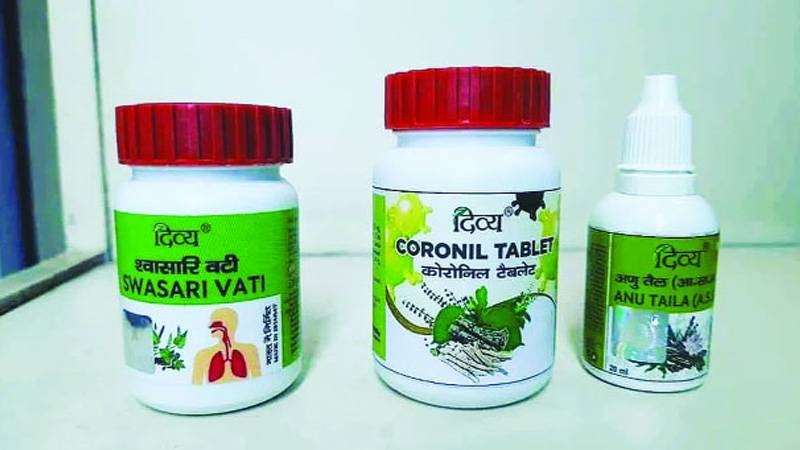
Patanjali Ayurved has launched the ‘Coronil and Swasari’ medicine with the claim that it has discovered a cure for COVID-19.
However, the ministry said the facts of the claim and details of the stated scientific study are not known to it.
Patanjali has also been asked for details of the sample size, sites and hospitals where the research study was conducted and the Institutional Ethics Committee clearance, the ministry said.
Yoga guru Ramdev‘s herbal medicine company Patanjali Ayurved on Tuesday claimed to have discovered a cure for Coronavirus but no medical authority could immediately vouch for the claim of ‘Coronil and Swasari‘ medicine curing the highly contagious disease within seven days.
The firm claimed that the two Ayurved-based medicines have shown 100 per cent favourable results during clinical trials on COVID-19 infected patients except those on a life support system.
The medicines have been developed by Patanjali Research Center, Haridwar and privately-owned National Institute of Medical Science, Jaipur following all protocols with clinically controlled trial-based evidence, Ramdev told PTI in a telephonic interaction with PTI.
“Patanjali first conducted clinical case study and conducted clinical control trials following all protocols of drug discovery,” he said. Sidestepping questions on the drug being approved by government agencies such as ICMR, Ramdev said clinical controlled study of these medicines was done in several cities including Delhi, Ahmedabad and Meerut and the RCT (Randomized Clinical Trial) controlled with placebo was conducted at Jaipur-based National Institute of Medical Sciences & Research.
The Corona kit, priced at ₹545, would be available pan India within a week. The kit will have medicines for 30 days. This is not an immunity booster but a coronavirus cure, Ramdev said. Manufactured by Haridwar-based Divya Pharmacy and Patanjali Ayurved Ltd, the medicine is the result of a research partnership between Patanjali Research Institute and the Jaipur based National Institute of Medical Sciences. Ramdev said the medicines can be ordered online through a mobile app from next Monday.
The COVID-19 therapy suggested by Patanjali is applicable for adults ranging between 15-80 years of age whereas children are advised to take half the dosage prescribed for adults. Patanjali’s anti-COVID tablet, Divya Coronil Tablet consists of Giloy, Tulsi and Ashwagandha and is prescribed to be taken thrice a day with hot water 30 minutes after breakfast, lunch and dinner.