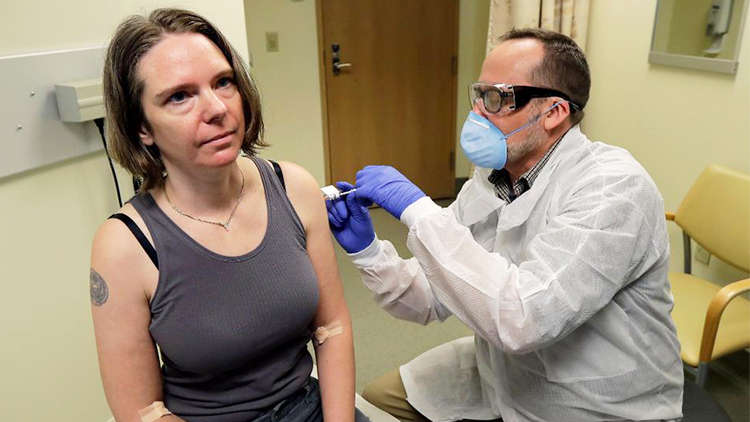
The US has begun the phase one clinical trial of an experimental vaccine designed to protect against the coronavirus disease, the tests are supported by a global coalition founded by India and Norway. A 43-year-old volunteer named Jennifer Haller from Seattle is the first person to get the vaccine.
The trial began at the Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The open-label trial will be tested on 45 healthy adult volunteers aged between 18 to 55 years for approximately 6 weeks.
The vaccine is called mRNA-1273 that was developed by the National Institute of Allergy and Infectious Diseases scientists, collaborating with the biotechnology company called Moderna, Inc., based in Cambridge, Massachusetts.
US president announced the news at a White House news conference on Monday, he said, “I’m pleased to report today that a vaccine candidate has begun the phase one clinical trial. This is one of the fastest vaccine development launches in history. Not even, close. We’re also racing to develop antiviral therapies and other treatments,”
According to the National Institutes of Health, the analysis will help to measure different doses of the investigational vaccine for safety and its ability to persuade an immune reaction in the participants. The investigational vaccine was established using a genetic platform called mRNA (messenger RNA). The investigational vaccine guides the body’s cells to express a virus protein that it is hoped will elicit a robust immune response. The mRNA-1273 vaccine has shown promise in animal models, and this is the first trial to examine it in humans.
Participants will be asked to return for follow-up between vaccinations for the span of one year after the second shot. The researchers will observe participants for common symptoms, such as soreness, fever, and other health issues. Volunteers were also asked to provide blood samples at specified time points, which examiners will test in the laboratory to detect and measure the immune response to the experimental vaccine.
A senior investigator at Kaiser Permanente Washington Health Research Institute, Dr. Lisa A. Jackson, said. “This work is critical to national efforts to respond to the threat of this emerging virus. We are prepared to conduct this important trial because of our experience as an NIH clinical trials center since 2007.”